All matter is composed of small particles known as atoms and molecules. Since different types of atoms and molecules have different properties, different types of matter also have different properties. Thus, the properties of matter are determined by the properties of its constituent atoms and molecules.
In this article, we will study in detail How Molecules are Formed?
1. Molecules- Definition
A molecule is formed when two or more atoms chemically bond together.
- A molecule represents a unit of a chemical compound.
- A molecule specifies the exact number of atoms in a unit of substance.
1.1 Types of Molecules
Molecules of elements are made up of atoms of only one kind of element. The chemical combination of atoms of the same element can form them.
For example, the molecule of Oxygen (O2) is composed of two oxygen atoms. Similarly, Nitrogen (N2) and Neon (Ne) are also composed of two and ten atoms respectively.
Molecules of compounds are made up of atoms of two or more different elements chemically bonded together. These compounds are formed by the combination of elements through chemical reactions. The chemical formula of a compound represents the ratio of atoms of each element in a molecule of that compound.
For example, water (H2O) is a compound made up of 2 hydrogen atoms and 1 oxygen atom, and carbon dioxide (CO2) is a compound made up of 1 carbon atom and 2 oxygen atoms.
Also Refer: How does an atom differ from a molecule?
2. How are Molecules Formed?
A molecule is formed up of several atoms that combine chemically.
These atoms are bonded together by certain forces of attraction. Atoms of the same or different elements can bind together to form molecules.
2.1 Why do Atoms form Molecules?
Atoms form molecules for a variety of reasons, but the most common reason is to achieve a stable electron configuration.
The stable electron configuration can happen when the atoms in a molecule are held together by chemical bonds- covalent, ionic, or metallic.
i. Covalent bond: In a covalent bond, atoms share electrons in order to achieve a stable electron configuration.
For example- water (H2O), methane (CH4), and carbon dioxide (CO2).
ii. Ionic bond: In an ionic bond, atoms transfer electrons to or from each other, resulting in the formation of ions. These ions are then held together by the electrostatic force of attraction between the positively and negatively charged ions.
iii. Metallic bond: This type of bonding occurs when atoms of a metal lose one or more of their electrons and become positively charged ions. These ions are free to move around the metallic structure. They are held together by the electrostatic attraction between the positively charged ions and the mobile electrons.
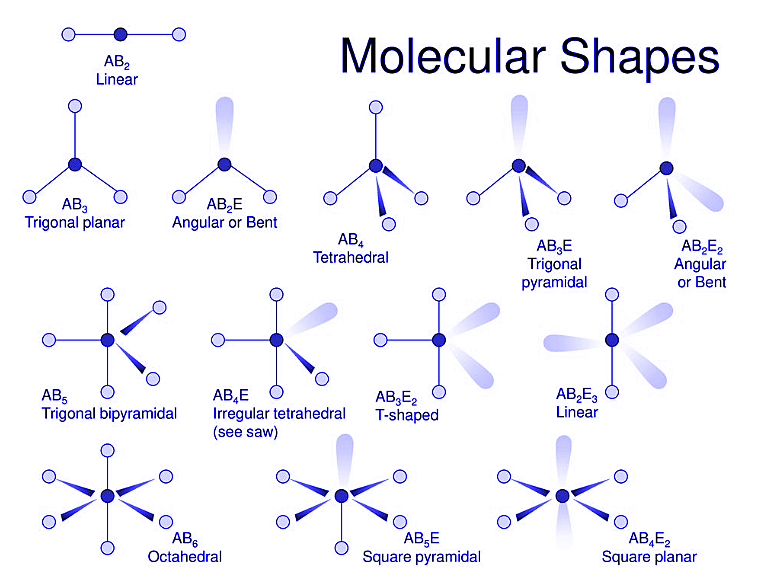
2.2 The shape and structure of Molecules
The chemical composition and bonding of atoms determine the shape and structure of a molecule. These characteristics are essential for determining the chemical and physical properties of the molecule.
The structure of a molecule refers to the arrangement of its atoms in a three-dimensional space, and it can be described in terms of bond lengths and bond angles.
The chemical bonds between atoms determine the molecule’s structure.
For example, a molecule with a single bond between its atoms will have a different structure than a molecule with a double bond.
The structures of molecular compounds can be linear, bent, trigonal planar, tetrahedral, and octahedral.
Key Takeaways
- A Molecule is the smallest particle of a substance that retains all of its physical and chemical properties.
- A molecule is composed of one or more atoms.
- They have a chemical bond between atoms.
- Sometimes the atoms are all from the same element.
- A compound is formed when two or more atoms of different elements combine together.
FAQs
How do atoms form molecules?
Atoms come together to form molecules through chemical reactions, which involve the combination, separation, or rearrangement of atoms. The atoms in a molecule are held together by chemical bonds, which can be covalent, ionic, or metallic.
What types of chemical reactions lead to the formation of molecules?
There are several types of chemical reactions that lead to the formation of molecules, including synthesis reactions, decomposition reactions, and displacement reactions.
– In a synthesis reaction, two or more atoms or molecules come together to form a new compound.
– In a decomposition reaction, a single compound is broken down into two or more simpler molecules or elements.
– In a displacement reaction, an element in a compound is replaced by another element.
How can chemical bonds affect the properties of molecules?
The chemical bonds between atoms determine the shape, structure, and properties of a molecule. Covalent bonds, for example, result in the formation of molecules with strong intermolecular forces, while ionic bonds result in the formation of molecules with strong electrostatic forces. These chemical bonds also determine the reactivity of a molecule and, therefore, its ability to participate in chemical reactions.
Why do some elements form molecules while others do not?
Some elements like Helium and Neon are chemically unreactive, they do not form compounds or molecules with other elements. These elements exist as single atoms and are called noble gases. On the other hand, some elements like Nitrogen and Oxygen can form molecules with other elements, as they have a tendency to share or transfer electrons with other atoms in order to achieve a stable electron configuration.
Can molecules be broken down into their individual atoms?
Yes, molecules can be broken down into their individual atoms through a process called chemical decomposition. This is achieved by exposing the molecule to certain conditions, such as heat, light, or chemical reagents, which cause the chemical bonds between the constituent atoms to break apart.
