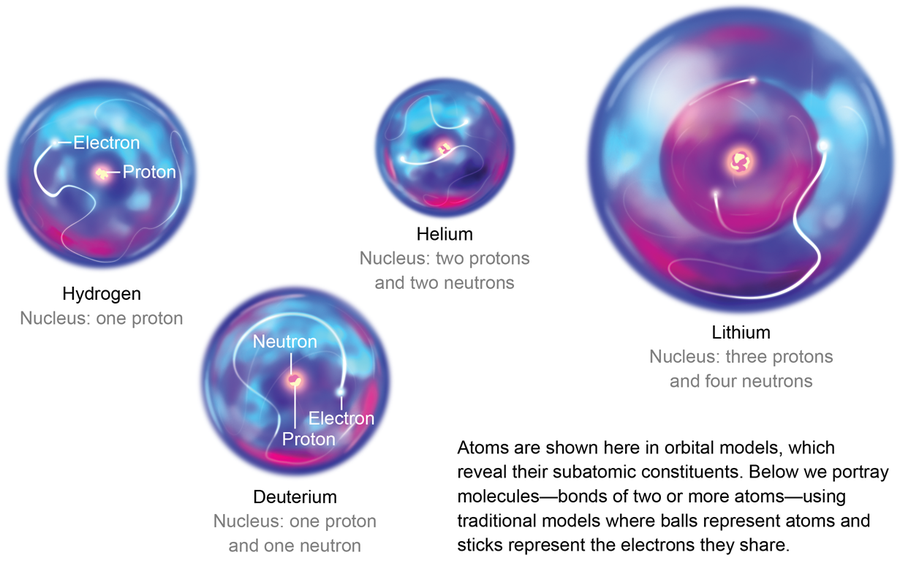
The first “atoms” in the universe were not atoms at all—they were just nuclei that had not found electrons yet. The simplest nucleus, that of common hydrogen, is a bare proton with no frills. When the universe banged into existence, energy was rampant. Everything was smashing into everything else. Protons and neutrons often collided, and some formed larger nuclei, such as that of deuterium (containing a proton and a neutron), as well as helium nuclei with two protons and two neutrons. Various other arrangements of protons and neutrons also formed, but because the identity of an atom is determined by its number of protons, all these other conglomerations were basically just different versions of hydrogen, helium and traces of lithium.
Of these three, helium was the first to begin forming “real” atoms. An atom is more than a nucleus—it must also possess electrons. Helium nuclei were the first to gather a full purse of electrons en masse. Why not hydrogen or lithium? Well, helium is the first “noble gas” on the periodic table—the first atom with enough electrons to completely fill the available slots in its electron shell. Thus, if electrons are the currency of chemistry, helium is the master pilferer of the periodic table. In a modern laboratory, it takes more energy to steal an electron from helium than from any other element. And the energy required to remove a second electron is more than twice what it takes for the first. In the early universe, once helium nuclei began to find electrons, they filled the coffers of their electron clouds well before the hydrogen nuclei could begin to catch up and before enough lithium nuclei were even present to collect all three of their desired electrons.
Credit: Elena Hartley
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
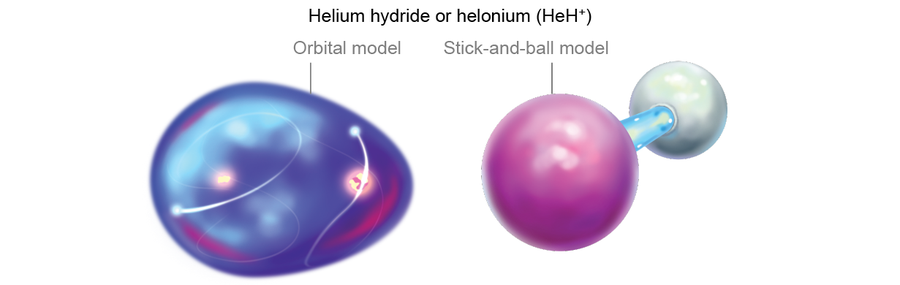
The rest of the matter in the universe at that time was still largely composed of lone protons, which were starting to feel the effects of being bereft of an electron. They began slowing down and looking for oppositely charged partners to make them electrically neutral. But catching free electrons for themselves was difficult, so the protons turned to helium, which already had some. Although helium is loath to share, it kept running into persistent hydrogen nuclei all the time. The collisional pressure eventually led a few helium atoms to share their electrons with protons. Thus, the first chemical bonds were formed. The new compound of helium and hydrogen was called helium hydride or helonium (HeH+), the very first molecule (of any sustained abundance) in the universe.
Credit: Elena Hartley
That helium was the first element to bond is surprising because in our current age, we think of helium as the least likely element to link up with others—the satisfied noble gas with just the right number of electrons. But in the early universe, helium was the only game in town—the only bank with electrons to lend.
This story has stood on solid theoretical ground for decades, but it has long lacked observational corroboration. HeH+ cannot form on Earth, except in labs, and for decades it went undetected in space. Last year, however, astronomers announced that they had observed this molecule for the first time, lurking in the funeral pyre of a dying star. A 40-year search had paid off, and a new and vital piece was added to our picture of how the early universe took shape.
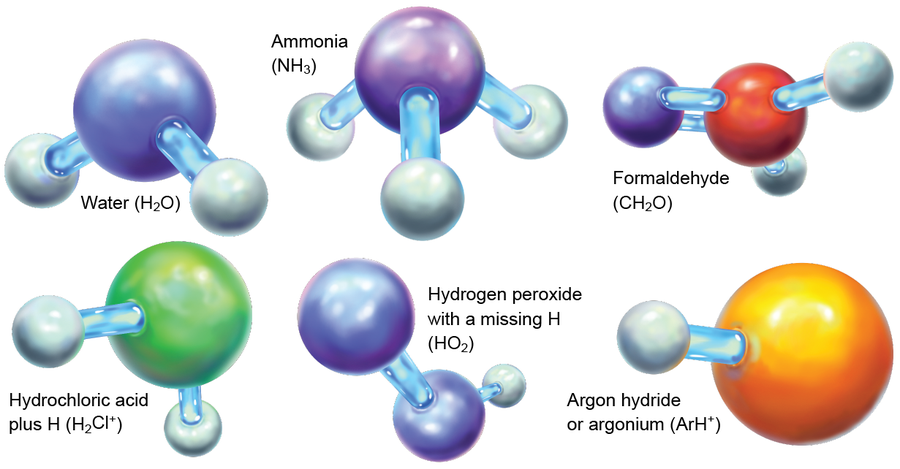
HeH+ now joins the ranks of extraterrestrial molecules; so far scientists have detected more than 200 molecular species in space. This study of chemistry beyond Earth—astrochemistry, as we practitioners like to call it—is aimed at clarifying what molecules are present in space, how they form, and what their evolution means for observational and theoretical astrophysics. Many of the known astromolecules, including water, ammonia and formaldehyde, are common here on Earth. Others are terrestrially bizarre, such as hydrochloric acid with an extra proton and hydrogen peroxide with one of its hydrogen atoms amputated. Charged molecules, systems with unpaired electrons and strange arrangements of atoms in otherwise common molecules have also been observed. We have even seen molecules containing the so-called inert noble gases, such as ArH+ (a combo of argon and hydrogen) and the newly documented HeH+.
Credit: Elena Hartley
Most disciplines of chemistry are focused on making the world safer, more efficient or more enjoyable for humans. Astrochemistry, however, looks at the most fundamental properties of molecules. It helps to define what bonding really is, how long molecules can remain intact and why certain chemical species are more common than others. By studying chemistry in environments so very alien compared with Earth—with temperatures, pressures and available ingredients quite different from what we are used to—we can find molecules that challenge our usual notions of how atoms interact and that bring us to a deeper chemical understanding. Ultimately we hope to learn how chemistry led to the ingredients that ended up in the planets in our solar system and eventually enabled life.
Where Was HeH+?
In a University of California, Berkeley, lab in 1925, T. R. Hogness (who later worked on the Manhattan Project) and teaching fellow E. G. Lunn found that mixing helium and hydrogen gas in the presence of an electric arc within a vacuum chamber could create different ions with different masses. Measuring the mass-to-charge ratio of molecules is the forte of the chemical discipline called mass spectrometry; the early implementation of this now common chemical technique showed that this mixture produced a transient mass-to-charge ratio of 5. That could only be HeH+. But keeping this noble gas molecule around long enough to study it proved exceptionally difficult, even in Hogness and Lunn’s controlled lab.
In the early universe, it would have been even more unstable because HeH+ is likely to let go of its proton on even the slightest contact with another atom. In this relationship, helium gives two electrons, whereas hydrogen gives none. Such uneven bonding (called dative bonding) is weaker than traditional covalent bonds, in which both atoms contribute more evenly.
In 1978 John H. Black, then at the University of Minnesota, was the first to argue that HeH+ could still be present in space. Black suggested that a good place to look was planetary nebulae, the puffed-out and highly energized matter created in a star’s death throes. In these clouds, a thin layer of ionized helium atoms is typically found in the presence of neutral hydrogen atoms; helium’s strong need for electrons could drive it to borrow one from hydrogen, creating a bond. Consequently, since the late 1970s astronomers and their chemist collaborators have been looking for HeH+ in myriad places, from the edge of the universe to supermassive stars. Yet for decades these searches found nothing, leading some to doubt the validity of HeH+’s role in jump-starting chemistry. Did helium really bond with H+? It seemed like it must have; there was nothing else to bond with back then. But if that were the case, then where was HeH+?
Molecular Fingerprints
While astrochemists were looking for HeH+ and coming up empty, researchers found many other molecules they were not expecting. They could not even identify some of them.
It began in 1919, when Mary Lea Heger was using the Lick Observatory on top of Mount Hamilton in Santa Clara County, California, to observe the behavior of a pair of orbiting binary stars, a twin system akin to the suns of Tatooine. What she saw was surprising.
Each molecule has its own arrangement of atoms and electrons and therefore absorbs light in a unique way. These “absorption features” give every molecule its own set of fingerprints, seen when astronomers separate incoming light into its constituent wavelengths—a process called spectroscopy. As Heger’s binary stars orbited their central point of gravity, the spectral features in each star’s atmosphere also shifted in wavelength (the Doppler effect).
But Heger also found some spectral fingerprints that were standing still as the stars moved around. She then looked at another binary star system and saw the same pattern. Follow-up work showed that these nonmoving features also showed up when telescopes were aimed toward single stars. The imprints must have been coming from molecules not around stars but in the vast, cold regions between them. The craziest part was that basically the same fingerprints were present for all observed stars and even for other galaxies. The signatures, dubbed diffuse interstellar bands (DIBs), were everywhere. Scientists scoured the documented spectral features of molecules on Earth, newly synthesized ones from labs and those observed in space through radio-telescopic fingerprinting. Nothing matched the DIBs—they were something novel.
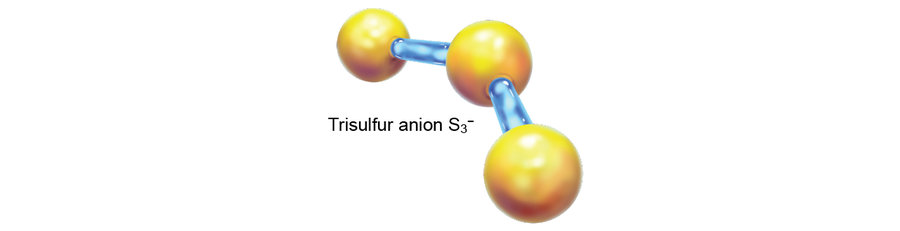
The late Harvard University professor William Klemperer, one of the foremost pioneers of astrochemistry, once suggested that the DIB signatures might belong to the trisulfur anion, S3–. When this proved untrue, he was so dejected that he wrote, “There is no better way to lose a scientific reputation than to speculate on the carrier[s] of the diffuse [interstellar] bands.” Hypotheses as to the provenance of the DIBs circulated through the decades, but none stuck—it was known as the longest-standing problem in spectroscopy.
Credit: Elena Hartley
One of the most intriguing hypotheses proposed polycyclic aromatic hydrocarbons (PAHs) as a DIB suspect. PAHs—hexagons of carbon atoms laid out in sheets—are the major component of soot, asphalt and graphite. They are unlikely to react with other molecules but do tend to stick to them. For astrochemists, the problem with PAHs is that their many varieties are so similar to one another that their spectroscopic fingerprints, or spectra, run together. It is like trying to spot the individual brushstrokes of Vincent van Gogh’s Starry Night instead of seeing the full painting—the many parts are subsumed by the whole. But the DIBs seemed to behave in a similar fashion. Could PAHs explain the DIBs?
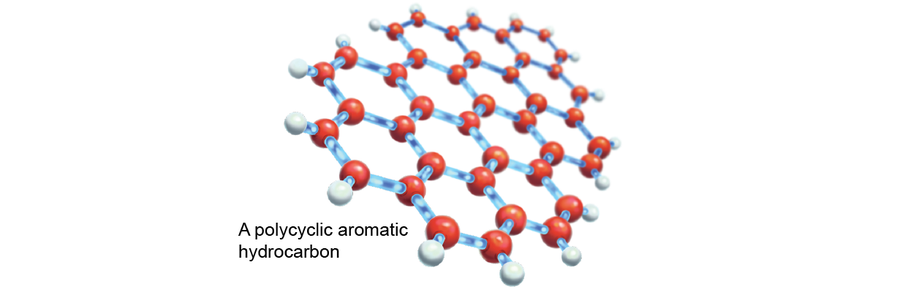
Credit: Elena Hartley
Such ideas have bounced around in astrochemistry circles since the 1970s, but one experiment forever changed how we think about carbon. Harry Kroto, who died in 2016, was at the University of Sussex in England in the 1980s and worked on a team to detect new molecules in space. He heard about an experiment by Robert F. Curl and Richard E. Smalley, both chemists at Rice University at the time, in which they had ablated an aluminum surface and found all kinds of new aluminum molecular clusters. When they substituted graphite (a so-called grand-PAH) for aluminum, a most bizarre molecule appeared: C60, 60 carbon atoms arranged like a soccer ball. In 1996 Kroto, Curl and Smalley were awarded the Nobel Prize in Chemistry for their roles in discovering the molecule, called buckminsterfullerene, or just fullerene (also known as a buckyball). Kroto was convinced that buckyballs were present in space and were likely to be the source of some DIB fingerprints. Only a few people believed him, though, and he and his colleagues moved on. Yet in 2010, a quarter of a century after their initial discovery in the laboratory, C60 and its cousin C70 were observed in the infrared in planetary nebula Tc1 in the constellation Cygnus. Whether these molecules were, in fact, related to the visible-wavelength DIBs was still undecided. Theoretical work suggested so, but scientists lacked confirming experimental data.
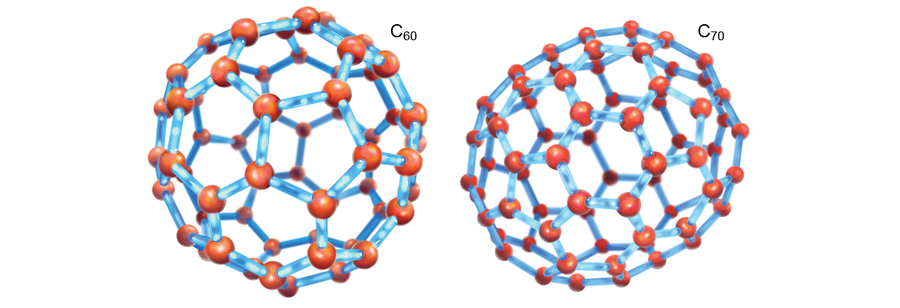
Credit: Elena Hartley
In 2015 the cation form of fullerene, C60+, was finally trapped in the lab, and scientists were able to conclusively measure its near-infrared spectrum. One, then two lines from this molecule matched known DIB wavelengths. Later, researchers showed that these fingerprints matched four or five DIBs. Then, in 2019, an international team led by Martin A. Cordiner of NASA’s Goddard Space Flight Center used the Hubble Space Telescope to examine the DIB wavelengths seen in the direction of 11 mostly red (older, bigger) stars and found that they matched the experimental data for C60+, confirming at last that this molecule’s fingerprints are responsible for some of the DIBs.
This discovery indicates that at least one type of molecule conclusively leaves its fingerprints all over interstellar space. Buckyballs are believed to evolve from PAHs, and their presence in space implies that their parent molecules must also be out there. Yet it was not until 2018 that researchers observed the fingerprints of a PAH-family molecule in space. The compound they saw, benzonitrile (C6H5-CN), is a rare aromatic hydrocarbon that is more easily detected than its relatives. And even more recently, scientists observed double-ring cyanonaphthalene molecules, revealing that larger PAHs are present as well.
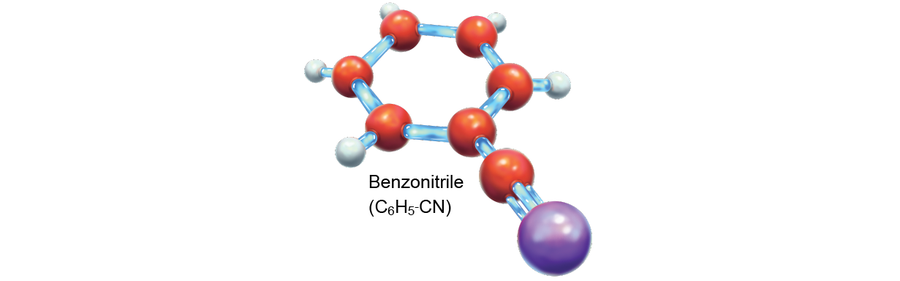
Credit: Elena Hartley
Discovery
Despite these breakthroughs, for a long time HeH+ remained elusive.
The first molecules would have dissipated fairly quickly after the earliest epochs. As the universe matured, expanded and cooled, the leftover hydrogen nuclei began to gather electrons of their own. At that point these now neutral hydrogen atoms presumably felt the positive charge on the HeH+ molecules. When the atoms and molecules collided, the relatively weak He-H dative bond broke, and a much stronger covalent bond between two hydrogens formed to create H2+. After that, the helium atoms were largely left alone.
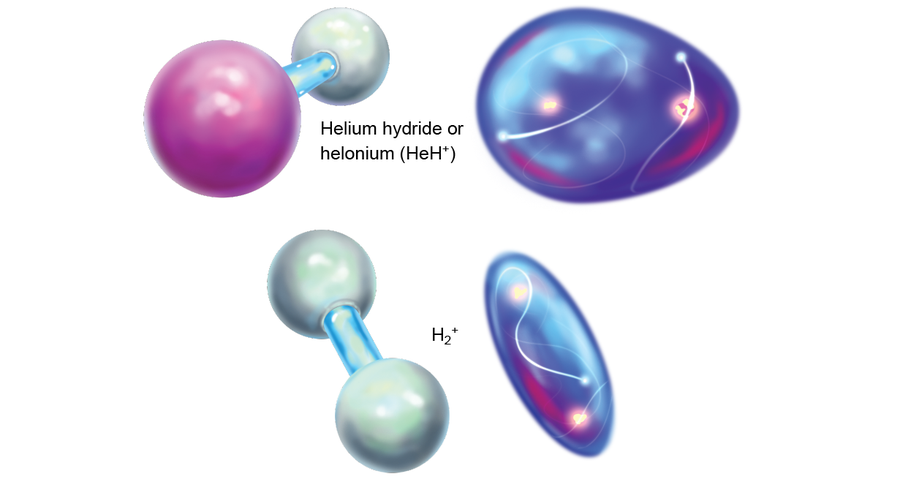
Credit: Elena Hartley
It might seem, then, that the brief existence of HeH+ was inconsequential, but that is far from the case. Models of potential chemical reactions in this period indicate that without HeH+ formation, H2+, and then neutral H2, would have come together much more slowly. Once H2 had been made, though, the entire tree of chemistry unfolded. Next came H3+, which begot CH+, which begot CH2+ and a cascade of other molecules. Eventually this chain led to water, ethanol and larger species. These processes are all the product of the unbalanced bonding in HeH+; without this initial relation, the universe would be a different place.
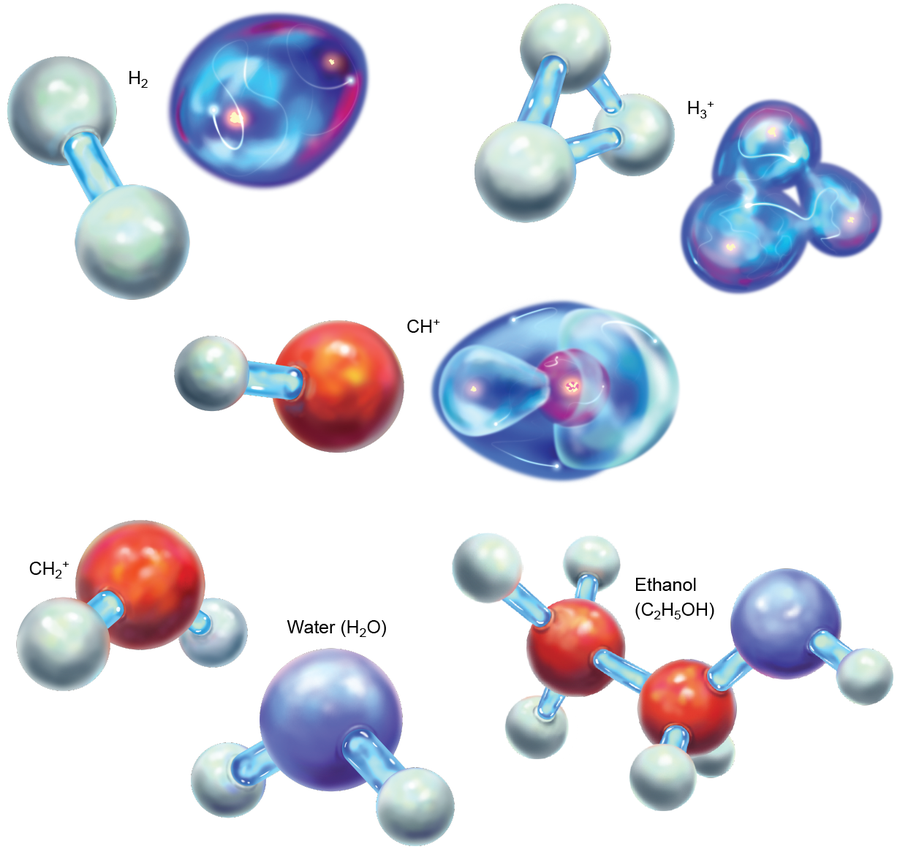
Credit: Elena Hartley
Still, by 2013 astrochemists were getting frustrated that HeH+ was nowhere to be found. But that year a hopeful sign came when researchers discovered the related noble gas molecule ArH+ in the Crab Nebula supernova remnant. Scientists focused the search for HeH+ in similar, superenergized environments. The larger problem, though, was that the spectra of HeH+ fell in the same region as fingerprints of the very first molecule ever observed in space, the CH radical. No telescopes had the power to separate these signatures.
Then along came the Stratospheric Observatory for Infrared Astronomy (SOFIA), a repurposed 747 jumbo jet with a hole cut in its side so an infrared telescope can look out. In May 2016 an international team used SOFIA, a joint project of NASA and the German Aerospace Center, for three nights of observations. The SOFIA scope has the resolution necessary to discern HeH+’s unique rotational-frequency fingerprint at 2,010.184 gigahertz. There, in the haystack of far-infrared data within another burned-out cinder of an exploded star in the planetary nebula NGC 7027, part of the constellation Cygnus, was the fingerprint that had gone missing for so long. This hellish place, with its high temperatures and energies, was not unlike the early universe. On April 17, 2019, a team led by Rolf Güsten of the Max Planck Institute for Radio Astronomy in Bonn, Germany, published a report in Nature heralding the discovery of HeH+.
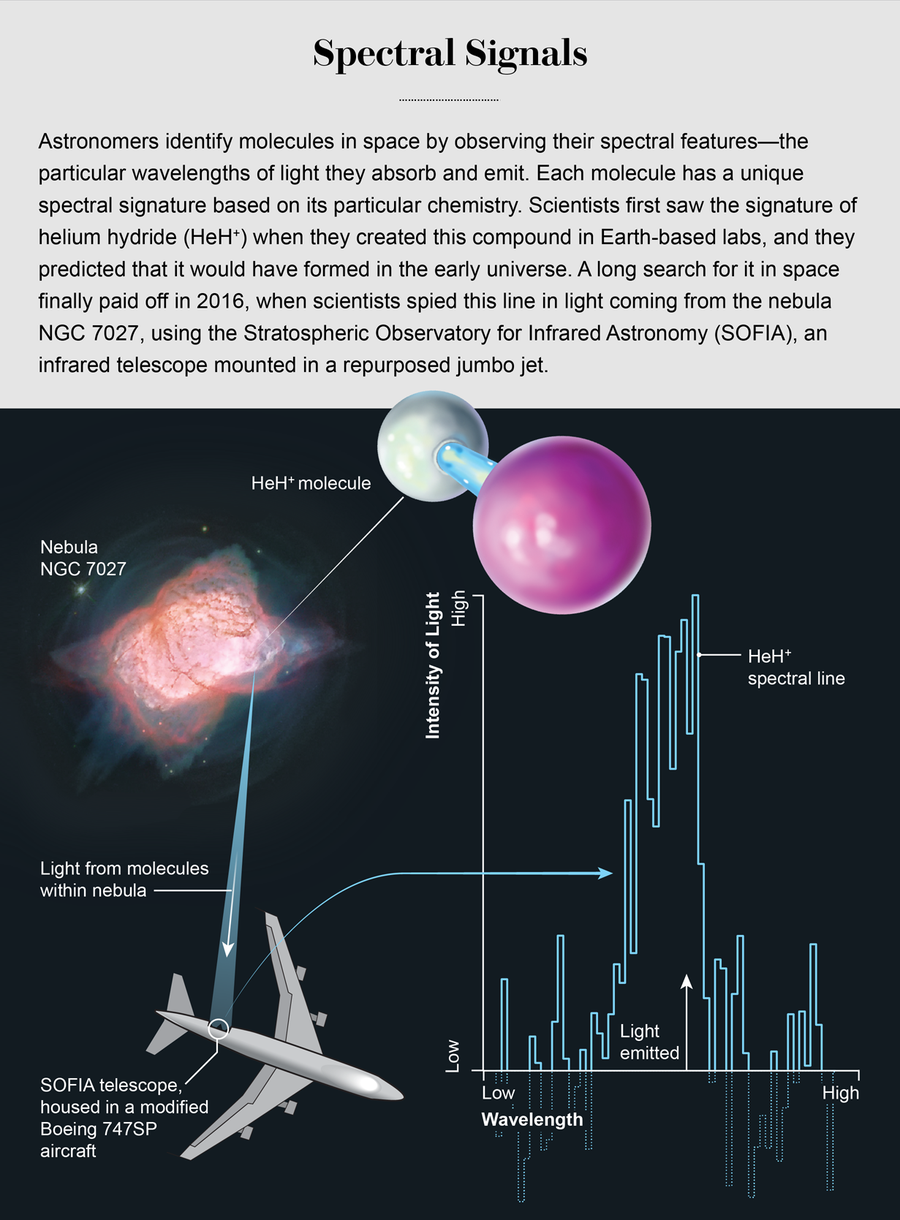
Credit: Elena Hartley (molecules) and Amanda Montañez (schematic); Sources: Hubble, NASA, ESA, processing by Jusy Schmidt (nebula); “Astrophysical Detection of the Helium Hydride Ion HEH+,” by Rolf Gusten et al., in Nature, Vol. 568; April 2019 (spectrum)
Granted, this sighting is not of primordial HeH+. We believe that the molecules Güsten and his colleagues observed were created much more recently. Nevertheless, the finding helps to constrain our knowledge of this compound. Scientists can now design better models of the universe as it existed when HeH+ was the only molecule in town. The discovery might also give us clues about where else this chemical may be lurking in space today, directing us toward other planetary nebulae or even other regions of space that are so far away they correspond to earlier epochs of time, going back to the edge of the universe.
Harder Questions
This is an exciting time in astrochemistry. Three grand questions have been conclusively answered in quick succession. Scientists have observed the first molecule to form in the cosmos and identified the first fingerprints belonging to the mysterious DIBs, and they are finally elucidating PAHs from the blackness of space.
Additionally, lab simulations of interstellar conditions are showing how amino acids and nucleobases might have formed. Space telescopes such as SOFIA and Hubble, as well as the upcoming James Webb Space Telescope, promise to provide unprecedented spectral characterization of stellar objects where new, less common molecular fingerprints may be seen.
Now that we are finding answers to these known problems, other quandaries are popping up. Eventually astrochemists hope to tackle harder questions, such as “What are the rest of the DIBs?,” “What are the molecular origins of life?” and “What chemical mix is necessary for the formation of rocky planets rather than gas giants?” It was the sharing of electrons that created observable matter in the cosmos. When we have a deeper comprehension of these chemical processes, we can gain a finer-grain understanding of astrophysics and the overall history of our universe.

